Without Ian MacLachlan’s innovative delivery system, Moderna and Pfizer couldn’t safely get their mRNA vaccines into your cells. So why does hardly anyone acknowledge the Canadian biochemist’s seminal contributions—or pay a dime in royalties?
In the summer of 2020, as the pandemic raged, infecting more than 200,000 people a day across the globe, Pfizer CEO Albert Bourla and BioNTech CEO Uğur Şahin boarded an executive jet en route to the hilly countryside of Klosterneuburg, Austria. Their destination: a small manufacturing facility located on the west bank of the Danube River called Polymun Scientific Immunbiologische Forschung. Bourla and Şahin were on a mission to get the company to manufacture as many lipid nanoparticles as possible for their new Covid-19 vaccine, which was on a fast track to receive emergency authorization from the U.S. Food and Drug Administration.
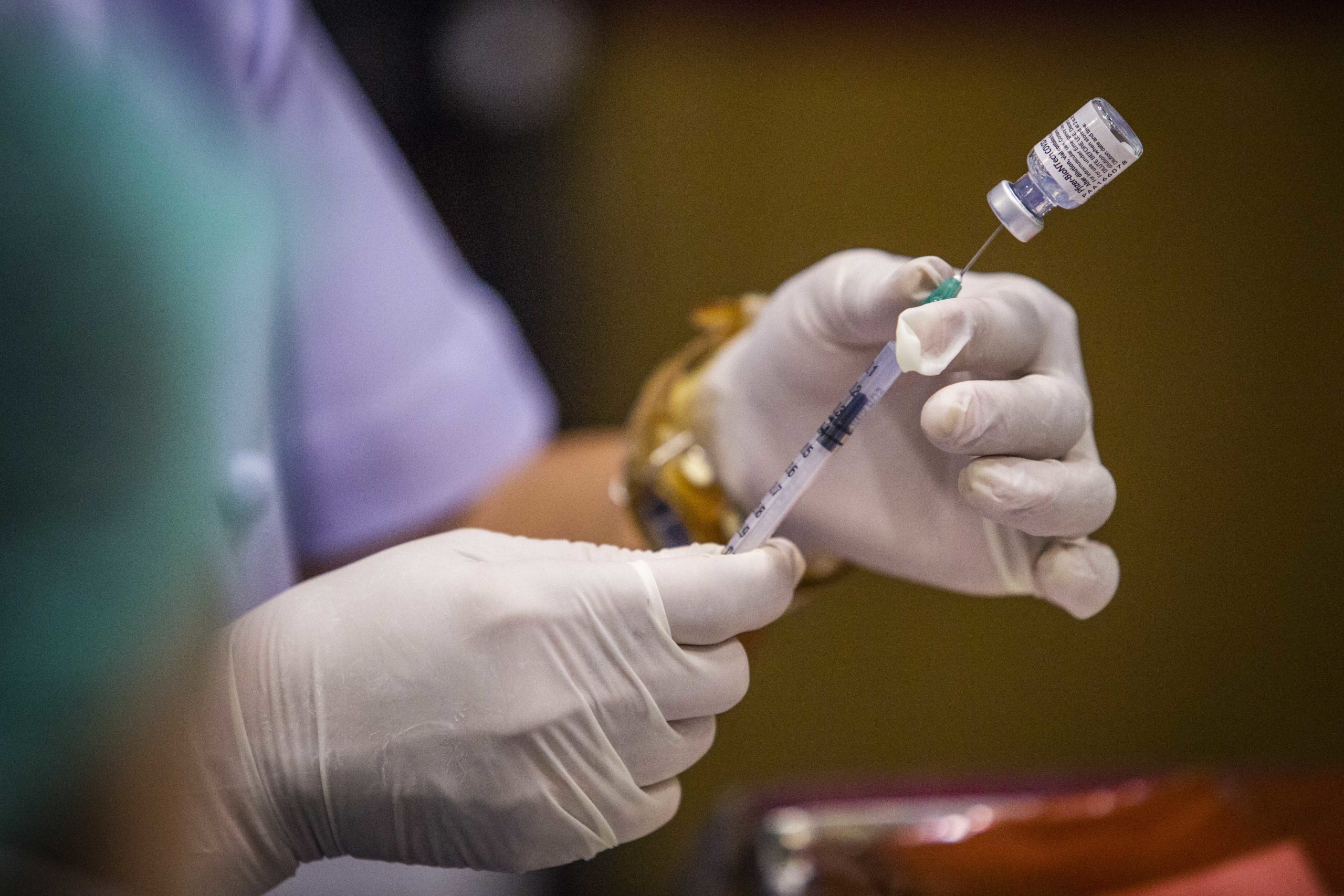
The Pfizer-BioNTech vaccine had been engineered with messenger RNA technology that instructs the body’s immune system to combat the coronavirus. But to get it safely into human cells, the mRNA needed to be wrapped in microscopic fragments of fat known as lipids. The Austrian manufacturing plant was one of the few places on earth that made the required lipid nanoparticles, and Bourla insisted Şahin go with him personally to press their case.
“The whole mRNA platform is not how to build an mRNA molecule; that’s the easy thing,” Bourla says. “It is how to make sure the mRNA molecule will go into your cells and give the instructions.”
Yet the story of how Moderna, BioNTech and Pfizer managed to create that vital delivery system has never been told. It’s a complicated saga involving 15 years of legal battles and accusations of betrayal and deceit. What is clear is that when humanity needed a way to deliver mRNA to human cells to arrest the pandemic, there was only one reliable method available—and it wasn’t one originated in-house by Pfizer, Moderna, BioNTech or any of the other major vaccine companies.
A months-long investigation by Forbes reveals that the scientist most responsible for this critical delivery method is a little-known 57-year-old Canadian biochemist named Ian MacLachlan. As chief scientific officer of two small companies, Protiva Biotherapeutics and Tekmira Pharmaceuticals, MacLachlan led the team that developed this crucial technology. Today, though, few people—and none of the big pharmaceutical companies—openly acknowledge his groundbreaking work, and MacLachlan earns nothing from the technology he pioneered.
“I look at the news, and 50% of it is vaccines—it’s everywhere—and I have no doubt the vaccines are using the technology we developed.”
“I just wasn’t going to spend the rest of my life dealing with it, but I can’t escape it,” MacLachlan says. “I open my browser in the morning and look at the news, and 50% of it is vaccines—it’s everywhere—and I have no doubt the vaccines are using the technology we developed.”
Moderna Therapeutics vigorously disputes the idea that its mRNA vaccine uses MacLachlan’s delivery system, and BioNTech, the vaccine maker partnered with Pfizer, talks about it carefully. Legal proceedings are pending, and big money is at stake.
Moderna, BioNTech and Pfizer are on their way to selling $45 billion worth of vaccines in 2021. They don’t pay a dime to MacLachlan. Other coronavirus vaccine makers, such as Gritstone Oncology, have recently licensed MacLachlan’s Protiva-Tekmira delivery technology for between 5% and 15% of product sales. MacLachlan no longer has a financial stake in the technology, but a similar royalty on the Moderna and Pfizer-BioNTech vaccines could yield as much as $6.75 billion in 2021 alone. In an ironic twist of fate, though, President Biden’s proposal to waive Covid-19 vaccine patents would make it unlikely that the intellectual property related to MacLachlan’s advances could be a source of riches.
Despite their denials, scientific papers and regulatory documents filed with the FDA show that both Moderna and Pfizer-BioNTech’s vaccines use a delivery system strikingly similar to what MacLachlan and his team created—a carefully formulated four-lipid component that encapsulates mRNA in a dense particle through a mixing process involving ethanol and a T-connector apparatus.
For years, Moderna claimed it was using its own proprietary delivery system, but when it came time for the company to test its Covid-19 vaccine in mice, it used the same four kinds of lipids as MacLachlan’s technology, in identical ratios.
Moderna insists the preclinical formulation of the vaccine was not the same as the vaccine itself. Subsequent regulatory filings by Moderna show its vaccine uses the same four types of lipids as MacLachlan’s delivery system but with a proprietary version of one of the lipids and the ratios “slightly modified” in a still undisclosed manner.
It’s a similar story for Pfizer and BioNTech. FDA documents show their vaccine uses the same four kinds of lipids in nearly the exact ratios that MacLachlan and his team patented years ago, albeit with one of those lipids being a new proprietary variation.
Not everyone ignores MacLachlan. “A lot of credit goes to Ian MacLachlan for the LNP [lipid nanoparticle],” says Katalin Karikó, the scientist who laid the groundwork for mRNA therapies before joining BioNTech in 2013. But Karikó, now a frontrunner for a Nobel Prize, is angry that MacLachlan didn’t do more to help her use his delivery system to build her own mRNA company years ago. “[MacLachlan] might be a great scientist, but he lacked vision,” she says.
Seven years ago, MacLachlan quit his position at Tekmira, walking away from his brilliant discovery and any potential financial rewards. Messy legal battles and political infighting within the biopharma industry over the delivery system had taken a toll on him. His emotions are complex. He may be overlooked, but he knows that he helped save the world.
“There’s a team of people who gave a great deal of their lives to the development of this technology. They gave their heart and soul,” MacLachlan says. “These people worked like dogs and gave the best part of themselves to develop it.”
• • •
Perched on a hilltop, Hohentübingen Castle towers above the town of Tübingen, Germany. In October 2013, MacLachlan, then the chief scientific officer of Tekmira Pharmaceuticals, trudged up the hill to the castle to attend a cocktail party at the first International mRNA Health Conference. During the evening, MacLachlan struck up a conversation with Stéphane Bancel, the CEO of an upstart mRNA company called Moderna Therapeutics. MacLachlan suggested Tekmira and Moderna collaborate using his innovative drug delivery system. “You are too expensive,” Bancel told him.
The exchange gave MacLachlan a bad feeling. So did the presence of a former colleague, Thomas Madden, who had been fired by Tekmira five years earlier. By this point MacLachlan had spent more than a decade working on his delivery system, yet people like Bancel seemed more interested in working with the London-born Madden.
The rivalry between these two scientists is the root of the controversy over the delivery technology that today’s Covid-19 vaccines rely on. MacLachlan and Madden met 25 years ago, when they worked together at a small Vancouver-based biotech called Inex Pharmaceuticals. With a Ph.D. in biochemistry, MacLachlan joined Inex in 1996, his first job after completing a postdoctoral fellowship in a gene lab at the University of Michigan.
Inex was cofounded by its chief scientific officer, Pieter Cullis, now 75, a long-haired physicist who taught at the University of British Columbia. From his perch there Cullis started several biotechs, cultivating an elite community of scientists that made Vancouver a hotbed of lipid chemistry.
Inex had a small-molecule chemotherapy drug candidate, but Cullis was also interested in gene therapy. His goal was to deliver large-molecule genetic material, like DNA or RNA, inside a lipid bubble so it could be safely ferried as medicine to the inside of a cell—something biochemists had dreamed about for decades but had been unable to accomplish.
Using a new method that mixed detergent with liquid, Cullis and his team at Inex successfully encapsulated small pieces of DNA in microscopic bubbles called liposomes. Unfortunately, the system could not consistently deliver bigger molecules, the type needed for gene therapy, in medically useful ways. They tried other approaches, including using ethanol, but didn’t succeed.
“We assembled all the LNP [lipid nanoparticle] pieces at Inex, but we didn’t get it to work” for genetic material, Cullis says.
Inex was a business, not a research lab, so it shifted its emphasis to the more promising chemotherapy drug. The gene therapy group was largely disbanded. MacLachlan ran what was left of it until, in 2000, he too decided to quit. Rather than let him completely walk away, Cullis persuaded MacLachlan to take the firm’s delivery assets and spin them out in a new company. Thus was born Protiva Biotherapeutics (MacLachlan became chief scientific officer), in which Inex retained a minority stake. MacLachlan recruited Mark Murray, now 73, a longtime American biotech executive with a Ph.D. in biochemistry, to be CEO.
It wasn’t long before two Protiva chemists, Lorne Palmer and Lloyd Jeffs, made a crucial discovery that led to a new mixing method. They put lipids dissolved in ethanol on one side of a physical T-connector apparatus, and, on the opposite side, genetic material dissolved in saltwater, then shot streams of the two solutions at each other. It was the moment they had been hoping for. The collision resulted in lipids forming a dense nanoparticle that instantly encapsulated the genetic material. The method was elegantly simple, and it worked.
In the midst of all this furious legal fighting, Hungarian biochemist Katalin Karikó showed up at MacLachlan’s door. Karikó was early to grasp that MacLachlan’s delivery system was key to mRNA therapies.
“The various methods that had been used previously were all highly variable and ineffective,” MacLachlan says. “Completely unsuitable for manufacturing.”
The team he led quickly went on to develop a new lipid nanoparticle made of four specific kinds of lipids. Though these were among the lipids Inex had also been using in its experiments, MacLachlan’s LNP had a dense core that differed significantly from the sac-like liposome bubbles developed by Inex. MacLachlan’s team had figured out the specific ratios of the four kinds of lipids that worked best relative to one another. Everything was dutifully patented.
Moderna and Pfizer’s Covid vaccines use a type of gene therapy based on the messenger RNA molecule. Protiva’s scientists, though, initially gravitated toward a different type of gene therapy using RNA interference, or RNAi. While mRNA instructs the body to create therapeutic proteins, RNAi aims to silence bad genes before they cause disease. With MacLachlan’s delivery system in hand, Protiva started collaborating with Alnylam, a Cambridge, Massachusetts–based biotech, to make RNAi therapy viable.
Meanwhile, MacLachlan’s old company, Inex, was imploding after the FDA denied accelerated approval to its chemotherapy drug. Inex fired most of its staff and then—despite having spun off Protiva only a few years earlier—looped back to drug delivery. It, too, started working in partnership with Alnylam. In 2005 Cullis quit, leaving none other than MacLachlan’s archrival Thomas Madden to run Inex’s delivery efforts.
In 2006, Protiva and Alnylam published a landmark study in Nature demonstrating the first effective gene silencing in monkeys. The study used the delivery system MacLachlan’s team had developed.
Alnylam went on to develop Onpattro, an RNAi drug used to treat nerve damage in adults with a certain hereditary condition. The drug would become the first RNAi medicine ever approved by the FDA. Regulatory filings show Alnylam used MacLachlan’s delivery system for Onpattro—with one exception. For one of the four kinds of lipids, Alnylam used a modified version it developed with Thomas Madden.
• • •
In October 2008, Mark Murray, the CEO MacLachlan had recruited to run Protiva, stood in a room at Tekmira Pharmaceuticals, a small publicly traded shell company he had just taken over. Like Protiva, Tekmira had been created by Inex, which had finally burned out a year earlier, but not before transferring all its remaining assets to Tekmira. Assembled before Murray were some 15 former Inex scientists who had come along in the deal, including Thomas Madden.
“Unfortunately, we are not going to be able to keep you guys any longer,” Murray told them.
Madden’s firing was one result of a massive legal brawl sparked by the fact that both Inex and Protiva had been working separately with Alnylam on drug delivery. The dispute would continue for years. In each iteration, Murray and MacLachlan would accuse Madden and Cullis of having improperly taken their ideas. Cullis and Madden, offended by the accusations, denied them. Sometimes they sued back, claiming Murray and MacLachlan had acted wrongly.
The first round of litigation resulted in a 2008 settlement that saw Protiva take over Tekmira, with Murray as CEO, MacLachlan as chief scientific officer and Madden soon fired. Despite the bruising, Madden and Cullis founded a new company in 2009 to continue working with Alnylam. Tekmira responded by suing Alnylam, claiming the Massachusetts biotech conspired with Madden and Cullis to cheaply gain ownership of the delivery system developed by MacLachlan. Alnylam denied wrongdoing and—of course—filed counterclaims, saying it simply wanted to work with Madden and Cullis, who had created an improved variation of one of the four kinds of delivery-system lipids.
That round of the legal brawl was settled in 2012, with Alnylam paying Tekmira $65 million and agreeing to assign dozens of its patents back to Tekmira. Those patents included ones for the improved lipid that Madden had developed for Onpattro. Under the deal, Cullis and Madden’s new company was granted a narrow license to use the MacLachlan delivery system to create new mRNA products from scratch.
Feeling defeated, MacLachlan quit Tekmira. He sold his stock, purchased a used Winnebago Adventurer for $60,000 and set off with his wife, two kids and their dog for a 5,200-mile road trip. “I was exhausted and demoralized.”
It was in the midst of all this furious legal fighting that Hungarian biochemist Katalin Karikó first showed up at MacLachlan’s door. Karikó was early to grasp that MacLachlan’s delivery system held the key to unlocking the potential of mRNA therapies. As early as 2006, she began sending letters to MacLachlan urging him to encase her groundbreaking chemically altered mRNA in his four-lipid delivery system. Embroiled in litigation, MacLachlan passed on her offer.
Karikó didn’t give up easily. In 2013, she flew to meet with Tekmira’s executives, offering to relocate to Vancouver and work directly under MacLachlan. Tekmira passed. “Moderna, BioNTech and CureVac all wanted me to work for them, but my number one choice, Tekmira, didn’t,” says Karikó, who took a job at BioNTech in 2013.
By this time, Moderna CEO Stéphane Bancel was also trying to solve the delivery puzzle. Bancel held discussions with Tekmira about collaborating, but talks stalled. At one point, Tekmira indicated it wanted at least $100 million up front, plus royalties, to strike a deal.
Instead, Moderna partnered with Madden, who was still working with Cullis at their drug delivery company, Acuitas Therapeutics.
In February 2014, MacLachlan turned 50. His life partner, Karley Seabrook, lured him to Vancouver’s Imperial theater, which was packed with friends and family. She surprised him in a wedding dress, and their two children greeted MacLachlan with cards that read WILL YOU MARRY MOMMY? Seabrook had never thought it important that they get married, but a brush with cancer had altered her perspective—and the wedding would alter his.
For the workaholic scientist, dealing with lawyers and endless corporate maneuvering had taken its toll. Feeling defeated, MacLachlan quit Tekmira in 2014. He sold his stock in the company, purchased a used Winnebago Adventurer for $60,000 and set off with his new wife, two kids and their dog for a 5,200-mile road trip across Canada.
“I was exhausted and demoralized,” he says.
With MacLachlan gone, CEO Murray renamed Tekmira, calling it Arbutus BioPharma, and decided the company should focus on creating hepatitis B treatments with New York drug development company Roivant Sciences. Yet he held on to the patents for the four-lipid drug delivery system.
Then Madden’s company, Acuitas, sublicensed the delivery technology to Moderna for the development of an mRNA flu vaccine. Murray was confident Madden had no right to do so, and in 2016 he gave notice that he intended to terminate Acuitas’ licensing agreement. Per custom, two months later, Acuitas sued in Vancouver, denying that it had violated any deal. On cue, Murray countersued, initiating a fresh round of legal combat. Importantly, though, this batch of lawsuits directly involved mRNA.
After battling for two more years, the parties settled. Murray terminated Thomas Madden’s license to MacLachlan’s delivery technology for any future medicines other than four products Moderna had already begun to develop (Murray also lost the rights to some of Madden’s technology). Murray and Roivant then created another company, Genevant Sciences, specifically to house the intellectual property related to the four-lipid delivery system and commercialize it.
Some companies were quick to come on board. Within a few months BioNTech CEO Şahin struck a deal with Genevant to use the delivery system for five of BioNTech’s existing mRNA cancer programs. The companies also agreed to work together on five other mRNA programs targeting rare diseases. There was no provision in the agreement about using the delivery technology for something completely unforeseen—something like Covid-19.
Moderna pursued a different strategy. It filed lawsuits with the U.S. Patent and Trademark Office seeking to nullify a series of patents related to MacLachlan’s delivery system, now controlled by Genevant. But in July 2020, as Moderna was pushing its vaccine through clinical trials, an adjudicative body largely upheld the most important patent claims. (Moderna is appealing.)
After the Moderna and Pfizer-BioNTech vaccines were authorized, Drew Weissman, a prominent mRNA researcher at the University of Pennsylvania, concluded in a peer-reviewed journal that both use delivery systems that are “similar to the Alnylam Onpattro product” but with a proprietary version of one of the lipids. Weissman noted both companies were using T-junction mixing.
Thomas Madden worked on the Pfizer-BioNTech vaccine delivery system and says he used enhanced versions of two of the four kinds of lipids. Madden says neither Onpattro nor the Pfizer-BioNTech vaccine would have been green-lighted by the FDA without his team’s improvements to the lipids.
MacLachlan dismisses the new variations as “iterative innovation.”
In a written statement to Forbes, Ray Jordan, Moderna’s corporate affairs chief, stated, “I can confirm that we did take a license to Tekmira’s IP for certain of our older products. But our newer products (including the Covid vaccine) have moved on with new technology.”
BioNTech declined to comment. Mikael Dolsten, Pfizer’s chief scientific officer, says the Pfizer-BioNTech vaccine is fully covered by patents and that in creating the first authorized mRNA product, Pfizer modified the delivery system to produce 3 billion doses annually.
“It’s different to have a process that may work for a very small scale than a large scale, and some of the assumptions that may look similar are based on how the scientific field evolved and [on] contributions from many different sources,” Dolsten says. “One needs to be careful in assuming that [if] things have similar names and similar molar ratios, it means it’s the same thing.”
Genevant declined to comment, but it could be fighting an uphill battle. In May, the Biden Administration backed waiving intellectual property protection on Covid-19 vaccines. Ironically, such a move might benefit, not hurt, Moderna, BioNTech and Pfizer by preventing Genevant from making any claims on their gigantic vaccine cash pile.
That’s just as well for Ian MacLachlan, whose role in what may be the most important medical advance in a century has been all but erased by the biotech industry.
“I definitely feel I made a contribution,” he says. “I have mixed feelings because of the way it’s being characterized, and I know the genesis of the technology.”