The debate on why convalescent plasma therapy could be key for treating critical Covid-19 patients.
The race to create a vaccine for the coronavirus pandemic is underway, but the likelihood of developing, manufacturing and scaling-up a successful vaccine could take a number of months to years. Experts say that Covid-19 convalescent plasma (CCP), on the other hand, is a treatment option that could be rapidly scaled and widely available throughout South Africa.
“The biggest possibility at the moment is Covid-19 convalescent plasma because if we can get plasma from those people who have already had the virus and have got the antibodies, we can then use it in our severe patients,” explains Dr Anton Meyberg, a pulmonologist physician at Netcare Linksfield Hospital in Johannesburg. He adds that CCP will be used to treat patients who are not responding to baseline medication, especially those who develop acute respiratory distress syndrome, a severe lung condition and require mechanical assistance (like a ventilator) to breathe.
The use of CCP as passive immunization to treat viral infections is not new – it was first (and successfully) used over 100 years ago during the 1918 Spanish Flu pandemic when there were no other treatment options available.
“Plasma therapy is essentially the passive immunization by providing antibodies passively to the patient – as opposed to a vaccine which is active immunization. With a vaccine, you give somebody a small amount of dead virus and their body builds an immune response to fight back. Those antibodies become their immune mechanism. In passive immunization, you give someone else’s antibodies to a person to fight the virus,” explains Marion Vermeulen, a South African National Blood Service (SANBS) biomedical technologist who is also co-principal investigator for the South African PROTECT Covid-19 convalescent plasma trial.
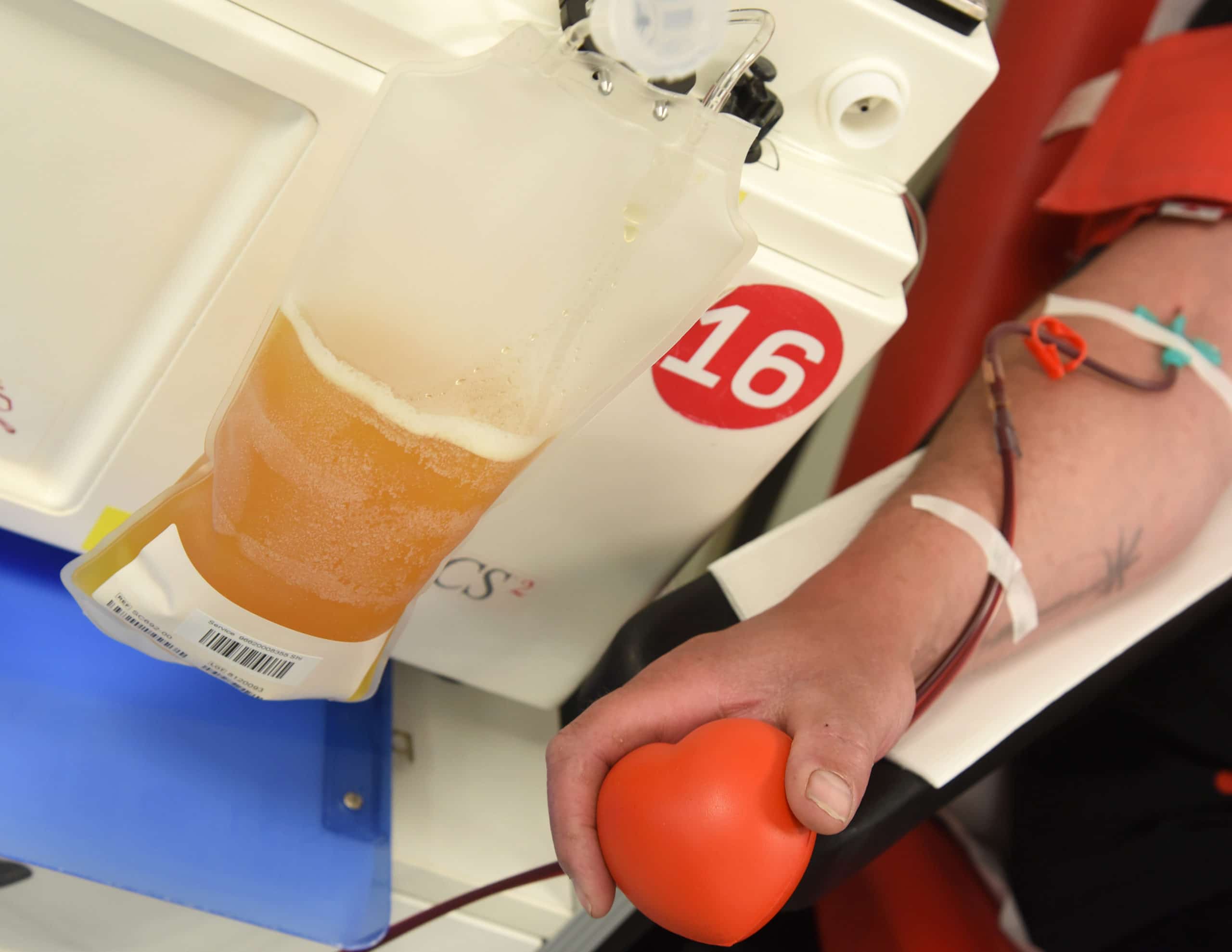
The SANBS collect non-Covid-19 plasma routinely as part of their transfusion service for the National Bioproducts Institute, South Africa’s fractionator. The process is identical for when a patient has recovered (or convalesced) from Covid-19, who have Covid-19 antibodies in their blood – antibodies contain proteins the body uses to fight off infections. After being symptom-free for 28 days, they can donate plasma via a procedure called apheresis. Each CCP donation has sufficient volume to be split into two products, so two Covid-19 patients can be treated with each CCP.
“We want to see that if we give these antibodies to patients, whether those antibodies will block the receptor that the virus uses to enter the cells and will therefore truncate the illness and hopefully give a milder disease,” says Vermeulen. “During plasmapheresis, blood is collected from the donor into an instrument that can centrifuge the blood. The white and red cells are returned to the donor during the procedure and only the plasma component is collected. Because red cells are not collected, a plasma donor can donate every two weeks. We’re also pathogen-reducing the product to further minimize the risk of HIV, hepatitis B and hepatitis C.”
All blood products have a small risk of transfusion transmissible infections even with the state-of-the-art testing performed, which means not everyone is eligible to donate CCP – people who have recovered from Covid-19 need to be between the ages of 18 and 65 and weigh at least 55kg and must have lived a safe lifestyle in the past three months. Unfortunately, women who have been pregnant before can’t enrol in the study: “When pregnant, women can develop human leukocyte antigen (HLA) and human neutrophil antigen (HNA) antibodies. With plasma transfusion, these antibodies have a small risk of causing a transfusion-related acute lung injury, or TRALI. Because Covid-19 is a lung disease, we don’t want to exacerbate an illness in someone who’s already got a lung disease,” she explains.
Currently, the SANBS has put the call-out for CCP plasma donors. They do not even have 1% of all the recoveries recorded on various South African Covid-19 statistic dashboards donating plasma. But as Covid-19 infection rates increase, they’re hoping to see an increase in the number of those willing to donate and potentially save many lives.
“The big worry at the moment is what happened in Europe and Latin America – they started the clinical trials too late. And they’re now at the tail end of the epidemic and they can’t get their numbers into the clinical trials and so they can’t determine if it actually works. In South Africa, we have a really unique situation because the pandemic started later here. We have the opportunity to enrol many patients… but we need to have sufficient product before we start the clinical arm of the study,” says Vermeulen.
– By Tiana Cline